Desmutting is used in chemical pretreatment after an alkaline etching process or a polishing process. Due to the removing effect ("etching") of these process steps, a more or less strong mix of different alloy components remains on the surface, depending on the material alloy.
This is often visible as a coating, but sometimes not.

Typical deposit formation after etching
However, it should always be removed so no stains or color changes are visible on the surface of the anodised component in the subsequent anodisation process. This task is performed by the desmutting, which removes the tarnish or oxide layers still present on the surface using suitable acids.
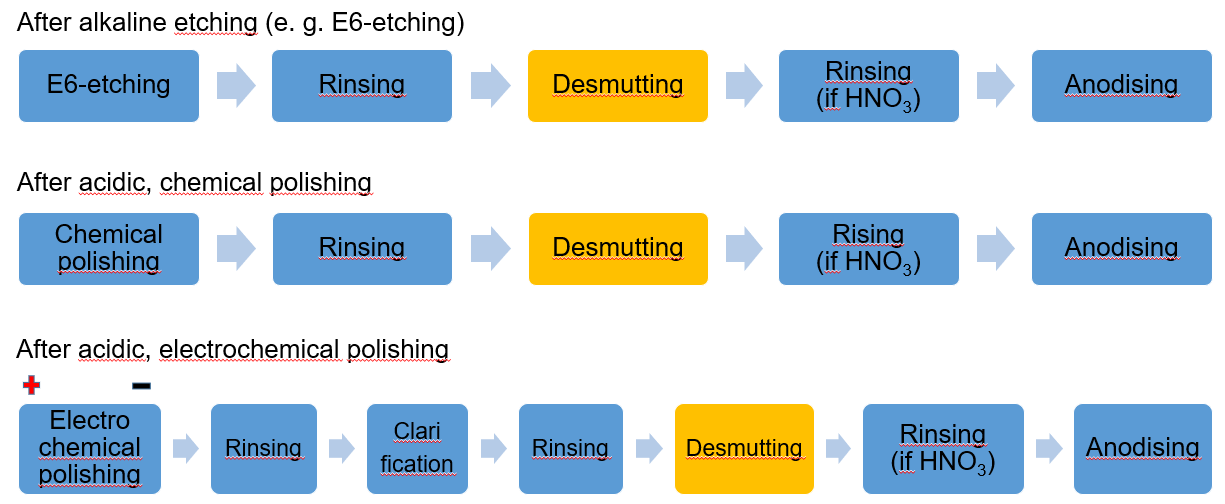
Use in practice
Previous techniques and further development
In the past, nitric acid was almost exclusively used for this purpose in a concentration of about 80 - 120 g/L, which has established itself as an excellent desmutting agent. However, due to possible emissions (NOx) and nitrate contamination in waste water, the use of nitric acid for this process is restricted or banned altogether in many regions.
Today, electrolytes containing sulfuric acid, which are the main component in most anodising baths anyway, are usually used as a substitute.
As aluminium-containing sulfuric acid has to be regularly replaced by fresh acid during DC-sulfuric acid anodising, the use of this acid as a desmutting agent is an obvious choice. Thus, the desmutting process is usually carried out with aluminium-containing waste acid in a concentration of 160 - 200 g/L sulfuric acid. This measure also allows the waste acid to be reused in a sensible way. This alternative also offers another advantage. The subsequent rinsing process that is necessary when using HNO3 can be dispensed with.
Depending on which alloys are to be treated, the desmutting effect of this waste acid is often not sufficient to remove all residues. In this case, the addition of the Alfideox products helps
- Alfideox 75 (liquid, stabilised) or
- Alfideox 79 (powder)
Both peroxide-containing products are able to remove any remaining deposits in combination with the acid. While Alfideox 75 is used with sulfuric acid or used acid from the anodising bath, Alfideox 79 is able to remove even particularly stubborn deposits in combination with nitric acid.

Laboratory test with an AlMgCuPb alloy
The dark area on the left has already been desmutted in nitric acid - black deposits are still visible. On the right, with nitric acid and the addition of Alfideox 79, the surface is free of deposits.
Since peroxide-containing products are highly reactive compounds, one should avoid adding "catalysts" (e.g. in the form of metal dust) to the desmutting bath. These would artificially decompose the additives and thus reduce their effectiveness. The result is increased additive consumption.
In principle, it is also possible to proceed desmutting directly in the anodising bath by leaving the goods there for a certain time without current. However, one should bear in mind that the bath is always artificially contaminated by dissolved alloy components. This can also cause problems in the course of time. Therefore, separate pretreatment stages are always advantageous.
In the case of silicon deposits, however, even the best desmutting additives are of no help. The higher the silicon content in the casting alloy, the faster the etched surface is covered by a black deposit. With these alloys (e.g. AlSi12), a pickling process should therefore be dispensed with altogether. Existing Si based deposits can usually only be removed mechanically or by special etching.
