After anodising and colouring aluminium surfaces, it is particularly important to seal the created porous aluminium oxide layer well. This process can be carried out using various seal methods. One possibility is the hot sealing.
For this, fully demineralised water is used at a temperature of at least 96°C, to which a sealing additive is added. In principle, sealing in this water bath also works without a sealing additive, but this results in a visible deposit on coloured surfaces, which greatly reduces the decorative appearance of the final product. Sealing additives from the Alfiseal product group (e.g. Alfiseal 942, Alfiseal 959 or Alfiseal 975) avoid these problems.
Which chemical processes occur during hot sealing process?
The high temperature and the aqueous medium cause hydration of the aluminium oxide layer in the water bath. Here, the aluminium oxide formed during anodising swells and gradually transforms on top into so-called boehmite (AlO(OH) - aluminium oxide hydroxide).
Al2O3 + H2O ---> 2AlO(OH)
Through this swelling process within the pore, it is finally closed (sealed) and existing colourations are fixed as well. In a figurative sense, the process can be compared to the calcification of a pipe. However, the swelling process also takes place above the pores, which then becomes visible as a deposit on coloured surfaces if no sealing additive is used.
This hot sealing process usually takes 3 minutes/µm anodised layer thickness. A 20 µm thick anodised layer, as it is usual for architectural aluminium, therefore requires a total hot seal time of 60 minutes in the anodising plant.
With special additives (e.g. Alfiseal 938) it is also possible to reduce the sealing time.
Here is the schematic sequence (side view of anodised layer) of a hot sealing process:
Unsealed anodised layer

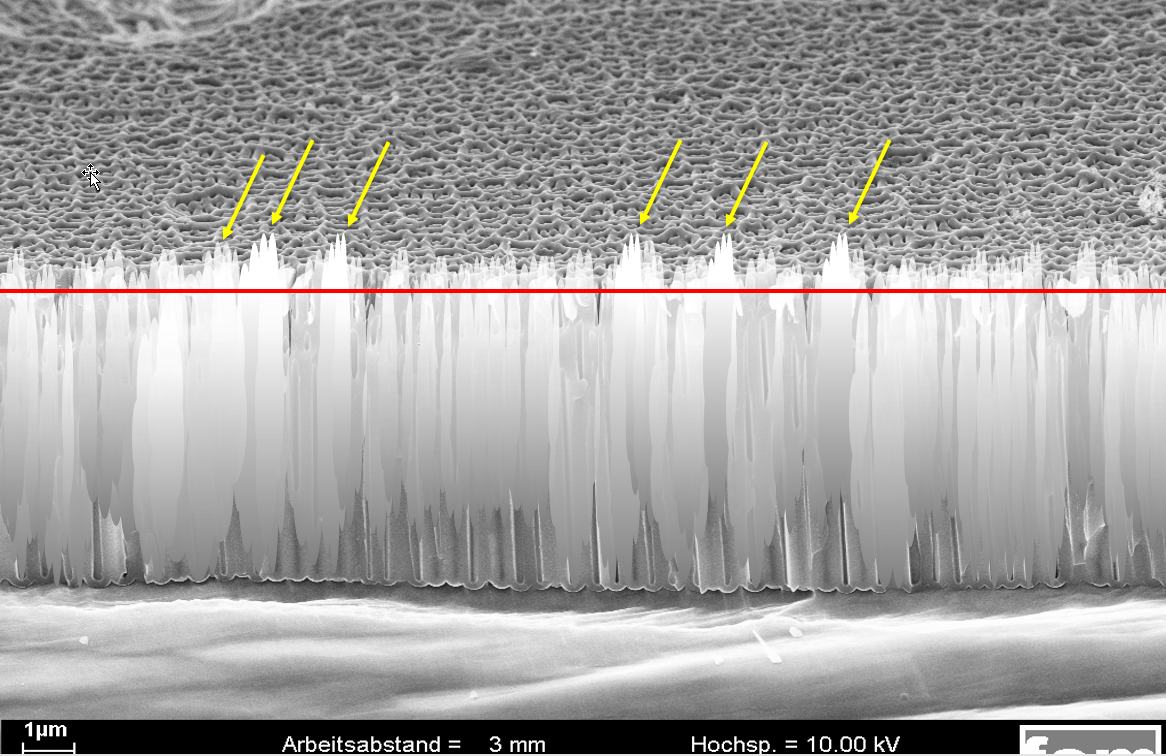
Alufinish simulation 1:
A sealing in fully demineralised water without sealing additive. Visible smut above the red line due to boehmite formed. The pores are closed. The sealing quality values are fine.
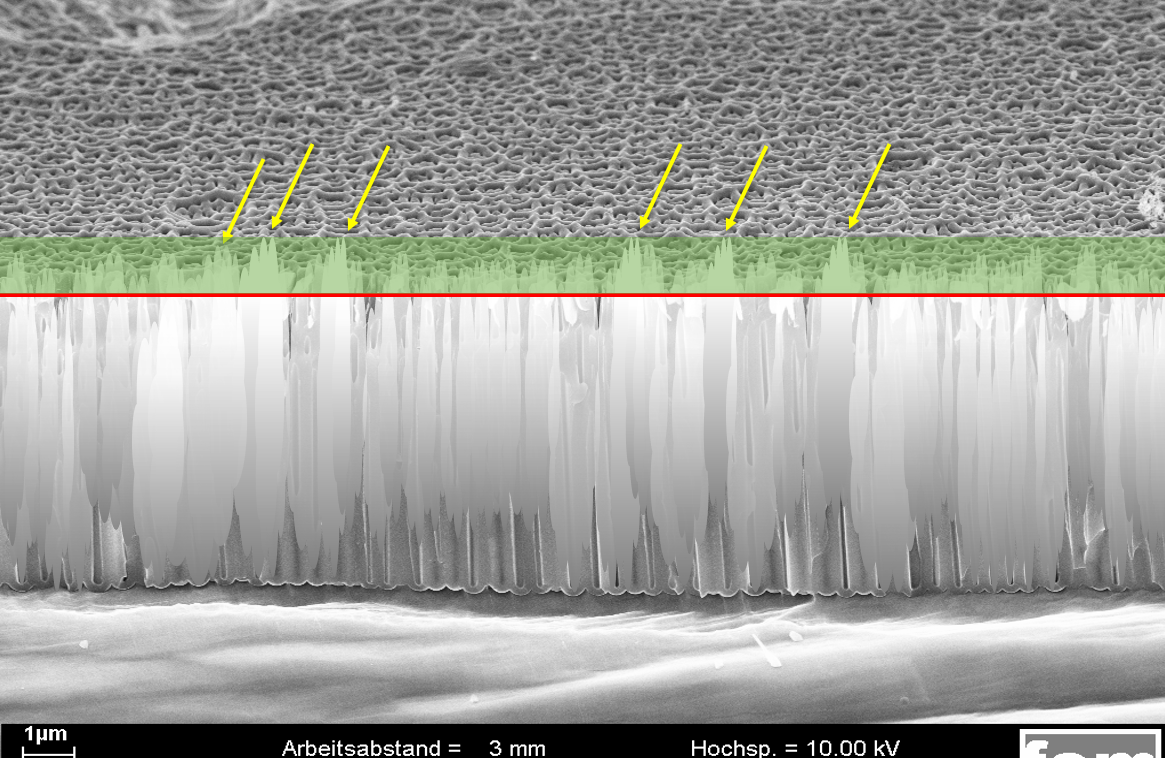
Alufinish simulation 2:
A sealing with correctly adjusted sealing additive. Only the visible deposit/smut above the red line is removed (green area). The surface is free of residues and the pores are closed. The sealing quality values are excellent.
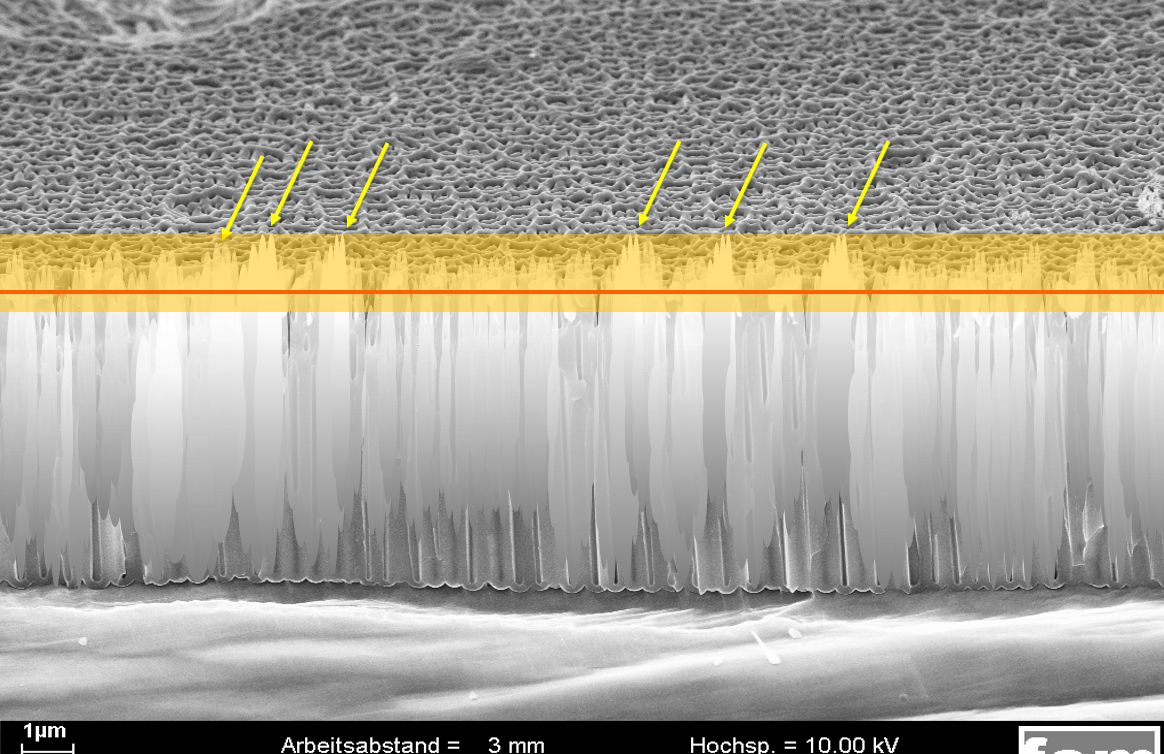
Alufinish simulation 3:
A sealing with incorrectly adjusted sealing additive. The visible smut/deposit above the red line is removed but also a part inside the pores (orange area) is removed. The surface is free of residues, but the pores are no longer completely closed. The sealing quality values deteriorate.
(Picture source SEM-image: FEM, Schwäbisch - Gmünd)
The following points are therefore important for good sealing of decoratively anodised aluminium surfaces:
- The correct choice of sealing additive enables a clean and deposit-free surface with good sealing quality at the same time
- pH-value and concentration have to be correct in order to avoid layer attack (see Alufinish simulation 3).
- The correct sealing times and bath temperature have to be maintained to enable complete sealing.
- The best sealing additive is of no use if the pores of the anodised layer have not been rinsed sufficiently beforehand. Intensive rinsing in demineralised water (conductivity: < 30 µS/cm is recommended) is therefore strongly advised. Through intensive mass exchange, any remaining sulphuric acid and salts (aluminium, tin...) are removed from the pores and replaced by water.
- Correct rinsing before hot sealing also reduces the entry of harmful ions into the bath and thus increases its service life.
- The entry of sealing poisons (fluorides, phosphates, silicates...) have to be prevented so that a good sealing quality can be achieved at all. Silicates are not detected by the conductivity value. Therefore, it have to been checked whether the fully demineralised water is also free of silicates after regeneration.
Download the brochure now
Save this article as PDF
This article is also available as a PDF in English. Download now free of charge.
