The best pretreatment is ineffective if the rinsing technique is inadequate. Often the process baths are elaborately maintained and analysed, while the equally important rinsing baths are neglected.
Therefore, during chemical pretreatment, the attention should always be paid to the condition of the rinses between the process baths and, in particular, to the rinsing situation before and after corrosion protection treatment. Any residues from a final rinse can be deposited on the treated goods and thus lead to problems with the painted goods later.
Depending on the condition and composition of the corresponding rinse, the residues can be process chemicals, oils/greases, dirt and powder particles or even turbidity (e.g. from dead biomass).

Corresponding residues remain on the pretreated surface and then dry out to a greater or lesser extent in the subsequent adhesive water dryer, for example in the form of powder or crystal-like salt residues. They leave either visible or initially invisible traces. If this dried salt film is then powdercoated, ideally nothing is visible at first and she paint adhesion does not suffer.
If a boil test or wet adhesion test is subsequently carried out for quality control purposes, rinsing problems or residues under the paint usually quickly become apparent through visible blistering under the paint.


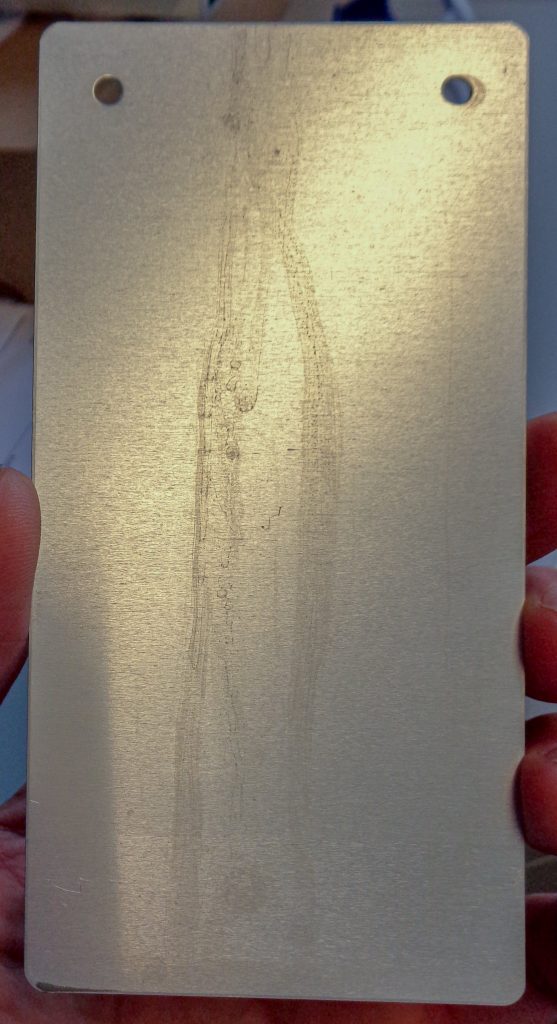
cause of the problem after paint stripping
In addition to purely visual defects (e.g. in the form of visible and tangible particles in the powder coating), residues can also lead to coating adhesion or reduced corrosion protection. If heavily loaded rinsing baths are not regularly replaced or at least partially replaced, subsequent rinsing and process baths will also be affected and artificially aged by the introduction of impurities via the goods. Suitable cascade rinses provide a remedy here.
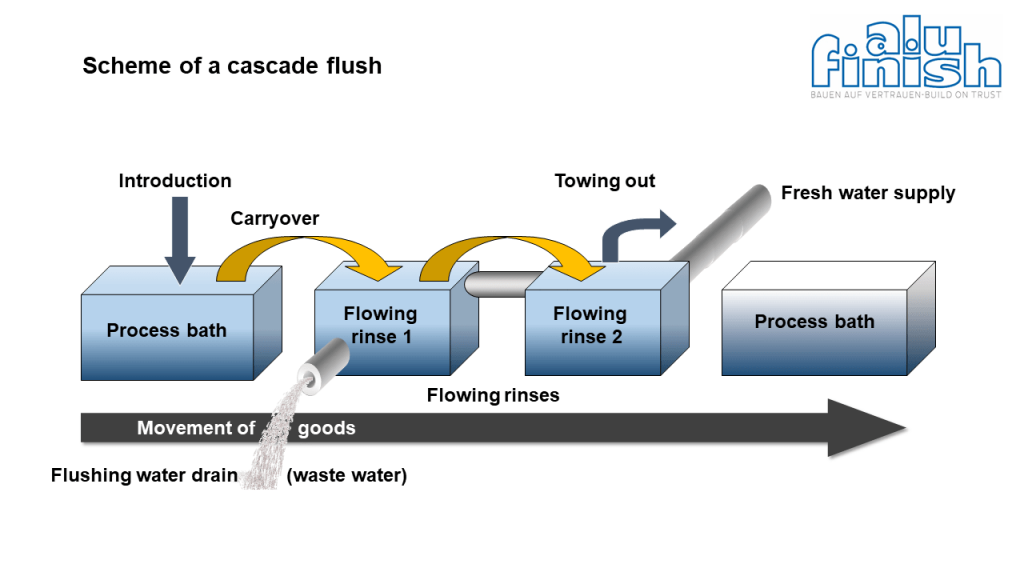
The counterflow principle (movement of goods to fresh water supply) of a cascade rinse always achieves the best (cleanest) water quality prior to the next process bath (or at the end of pretreatment). In the above diagram of a cascade flush, flowing rinse 2 has a better water quality than flowing rinse 1. Fresh water is fed into flowing rinse 2, while dirty water is fed from flowing rinse 1 to the waste water system. This minimizes the amount of dirt entering the downstream process bath.
However, even the best cascade rinsing system is of little use if it is not adequately maintained and adjusted to the required rinsing water quantities. Depending on the desired conductance, the correct ratio between inlet and outlet must be determined.
How can I assess the quality of the rinsing baths?
A glance at the corresponding rinsing bath can help here:
- Is the rinse bath very cloudy?
- Are there piles of foam on the surface of the rinsing bath?
- Are there oil-like residues on the surface of the bath?
- Are the plug-in sieves dirty?
- Are filamentous or slimy residues visible on pipework and tank walls, indicating microbiology
in the system? - Are powder coating residues visible on the bottom of the rinsing baths that have been
introduced into the pretreatment booth?
What is it about the conductance of the rinsing baths?
Especially with the chrome-free corrosion protection processes, which usually have only low conductance values compared to classic yellow chromating, a good rinsing technique is essential. Therefore, fully demineralised water is used before the process bath and for the final rinse (if rinsing is necessary at all). A maximum conductivity value of 30 µS/cm is usually used as a guide value. However, the water dripping off the pretreated goods is decisive and not only the water quality in the rinsing bath.

Why is it necessary to look at the dripping water?
It is possible that the water dripping off the pretreated substrates shows significantly higher conductivity values compared to the water quality in the rinsing bath. This is the case when residues from the process bath are rinsed out from corners and edges at this point. These then lead to an increased conductance and an additional load on the subsequent, chrome-free corrosion protection bath or can lead to salt deposits on substrate edges at the end of the pretreatment process. Therefore, the dripping water should ideally also be below 30 µS/cm.
An exception here is the so-called “no-rinse” corrosion protection process (e.g. Alficoat 748/3), which does not require a final rinsing process, but can optionally be followed up with fully demineralized water. The fog water that then drips off is often above 30 µS/cm conductance and thus corresponds to a highly diluted process bath solution. However, due to the special product composition, an increased conductivity value does not have a negative effect on the subsequent painting and corrosion protection here.

chain
What other causes are there for poor flushing technology?
In addition to chemical causes, there are often technical problems with the system. For example, clogged or misaligned spray nozzles or surge boxes in spray systems (waterfall systems) can contribute to flushing problems. Therefore, these should be checked at regular intervals and adjusted if necessary. Also, if the rinsing of hooks for profile fixation is insufficient, process bath residues can run over the surface at the contact point.

Incorrect fixing of test panels or profiles also often contributes to a poor test result (e.g. in the wet adhesion test). If the respective test specimen is fixed to the product carrier at one point, it can twist in such a way that the spray jet misses the surface. A two- or better three-point fixation of the material solves this problem. The problem is often recognized when the quality of the test sheets/profiles is inferior to that of the actual product.
The rinsing time and the exposure of the product to rinsing water are also decisive. If rinsing times are reduced, e.g. due to high fabric utilization, or if rinsing from one bath to the next takes place too quickly (e.g. immersion process, manual operation), this has a negative effect on the rinsing situation and cleanliness of the pretreated surface.

If mechanical damage occurs during subsequent use of a poorly rinsed component in conjunction with high humidity (rain, maritime climate, etc.), these defects begin to corrode more easily and can spread over the surface under the paint. The consequence of this will be a complaint by the end user. To avoid rinsing problems, the following points should be observed in particular:
- Adequate cleanliness in the rinses?
- Sufficient rinsing times?
- Quality of the dripping water?
- Observation of the goods after the individual process steps, as far as possible
- Are there blocked spray nozzles on each side?
- Spray nozzles adjusted if necessary?
- Are test plates protected against twisting, e.g. by two- or three-point fixing?
- Is there any microbiological contamination of the rinsing water?
